Come See Us
The Bludigo® Difference
FAST
DETECTION1
Within 4-9 minutes
post-IV injection
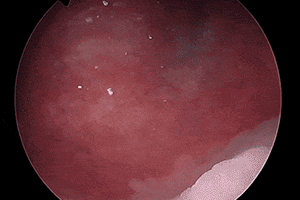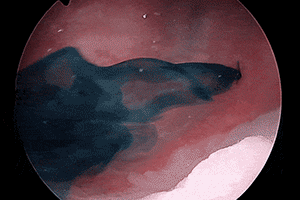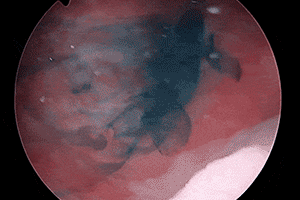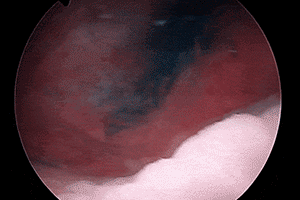
RELIABLE
VISUALIZATION2
Deep blue color with enhanced visualization of ureter flow3
WELL-ESTABLISHED
SAFETY PROFILE1
FDA-APPROVED INJECTABLE
INDIGO CARMINE1
The first and only FDA-approved injectable indigo carmine diagnostic dye
aWhen available, indigo carmine was considered the standard of care.4
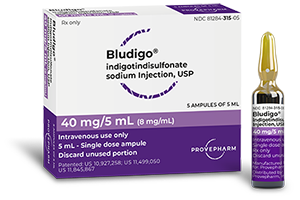
BLUDIGO® (indigotindisulfonate sodium injection, USP)
INDICATIONS AND USAGE
BLUDIGO® is a diagnostic dye indicated for use as a visualization aid in the cystoscopic assessment of the integrity of the ureters in adults following urological and gynecological open, robotic, or endoscopic surgical procedures.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
BLUDIGO® is contraindicated in patients with known hypersensitivity to indigotindisulfonate or any of its components.
WARNINGS AND PRECAUTIONS
Cardiovascular Reactions: Severe or life-threatening cardiovascular reactions including cardiac arrest, arrhythmia, asystole, atrioventricular block second degree, hypotension, elevation in blood pressure, bradycardia, and tachycardia have been reported. Closely monitor blood pressure and cardiac rhythm during and following the BLUDIGO® injection. Interrupt administration if reactions are observed.
Hypersensitivity Reactions: Serious anaphylactic reactions with hypotension, dyspnea, bronchospasm, urticaria, or erythema have been reported. Monitor patients for anaphylactic reactions and have emergency equipment and trained personnel readily available.
Interference with Oximetry Measurements: Anesthesiologists should be aware of the potential for artifactual reduction in SpO2 when anesthetized patients are administered BLUDIGO®.
USE IN SPECIFIC POPULATIONS
Renal Impairment: BLUDIGO® is not recommended for use in patients with eGFR<30 mL/min.
Pediatric Use: The safety and effectiveness of BLUDIGO® have not been established in pediatric patients.
Pregnancy and Lactation: Please consult the Full Prescribing Information before using BLUDIGO® in a patient that is lactating, pregnant, or may be pregnant.
RECOMMENDED DOSAGE
The recommended dose for BLUDIGO® is 5 mL given intravenously over 1 minute.
IMPORTANT ADMINISTRATION INSTRUCTIONS
- Monitor blood pressure and cardiac rhythm during and following the injection.
- Use immediately after opening ampule.
- Withdraw the contents of the ampule through a 5 micron or smaller filter straw/filter needle to ensure that the withdrawn solution contains no particulates. The withdrawn solution should be inspected visually for particulate matter and discoloration prior to administration.
- Do not administer with infusion assemblies used with other diluents or drugs.
- Discard any unused portion.
ADVERSE REACTIONS
Clinical Trial Experience: The most common adverse reactions (1%) associated with BLUDIGO® in clinical trials were: constipation, nausea, vomiting, abdominal pain, pyrexia, ALT increase, and dysuria.
Postmarketing Experience: The following adverse reactions have been identified following the use of indigotindisulfonate sodium injection products:
- Cardiovascular disorders: cardiac arrest, arrhythmia, asystole, atrioventricular block second degree, hypotension, elevation in blood pressure, bradycardia, tachycardia
- General disorders and administration site conditions: injection site discoloration
- Immune system disorders: anaphylactic reactions with hypotension, dyspnea, bronchospasm, urticaria, erythema
Please see the full Prescribing Information for additional Important Safety Information.
To report SUSPECTED ADVERSE REACTIONS, contact PROVEPHARM Inc at 1–833-727-6556 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
References: 1. Bludigo. Prescribing Information. Provepharm, Inc.; 2025. 2. Pickett CM, Seeratan DD, Mol BWJ, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database System Rev. 2023;8(8):CD003677. doi:10.1002/14651858.CD003677.pub6. 3. Data on file. PVP-10ICO1 clinical study report. Newark, NJ: Provepharm, Inc.; 2025. 4. Doyle PJ, Duecy E, Wood R. Sodium fluorescein as an alternative to indigo carmine during intraoperative cystoscopy. JMIG.2015
;22(3):S65.doi:10.1016/j.jmig.2014.12.147.